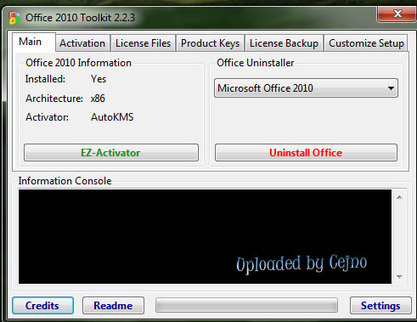
Office 2010 Toolkit And Ez Activator 2 2 3 Rat
Microsoft Toolkit is best tool for you to activate Microsoft Windows and Microsoft Office . It can activate includes Windows Vista, Windows 7 , Windows 8/8.1 and Windows 10. It also can activate MS Office 2007, 2010, 2013 , 2016 .
- Office 2010 Toolkit And Ez Activator 2 2 3 Rat Rod
- Office 2010 Toolkit And Ez Activator 2 2 3 Ratios
- Office 2010 Toolkit And Ez Activator 2 2 3 Rat Spray
How to Activate Windows or MS Office with Microsoft Toolkit
Home » MS OFFICE » Download Office 2010 toolkit 2.2.3 Aktivator Ms Office 2010. Jalankan softwarenya kemudian pada tab main klik EZ-Activator lalu tunggulah beberapa saat sampai proses selesai. Maka office anda akan menjadi full version. Yang perlu diperhatikan saat menajalankan software ini adalah matikan terlebih dahulu koneksi. Office 2010 Toolkit and EZ-Activator v2.2.3. This set of tools for managing licenses and activation of Office 2010. The result of all the functions displayed in the information in the console window. All operations are performed in the background, and a graphical interface is disabled to prevent multiple execution functions, since this could. Nov 07, 2013 Office 2010 Toolkit and EZ-Activator v2.2.3. This set of tools for managing licenses and activation of Office 2010. The result of all the functions displayed in the information in the console window. All operations are performed in the background, and a graphical interface is disabled to prevent multiple execution functions, since this could.
1. First you are required to disable the anti-virus system TEMPORARILY.
2. You open : Control Panel => System and Security => Change User Account Control Settings => Never notify
3. Download Microsoft Toolkit the Latest Version by these links :
4. Install, and Run the Software you download .
To Activate Windows you click icon Windows in this tool
Then, in the Activation tab, click ‘EZ-Activator’. Just in few seconds, your copy will be activated.
Note : If this tool can’t activate windows 10 , you can use tool to activate 7 , 8 , 8.1 , then you upgrade to windows 10 .
To Activate MS Office you click icon Office in this tool .

Then, in the Activation tab, click ‘EZ-Activator’. Just in few seconds, your copy will be activated.
Then you restart your pc .
You can watch this video to know how to use Microsoft Toolkit :
Change in Microsoft Toolkit .v2.6.5
-Added Label to show Microsoft Office Setup Version and Architecture on Customize Setup tab.
-AutoKMS no longer attempts activation / displays error when there are no KMS keys installed.
-Added more checks to Add Languages and do better filtering of Language Packs.
-Fixed Customize Setup failing if file extensions were not lowercase.
-AutoKMS skips running if a BSOD occurred within 30 minutes of the last Scheduled Task run.
-Scheduled Tasks now retain their last run date.
-Updated TAP Driver.
Microsoft Toolkit Toolkit .v2.6.5 can activate :
- Windows Vista all versions
- Windows 7 all versions
- Windows 8 all versions
- Windows 8.1 all versions
- Compatible with Windows 10
- MS Office 2003 all versions
- MS Office 2007 all versions
- MS Office 2010 all versions
- MS Office 2013 all versions
- MS Office 2016 all versions
Microsoft Toolkit is one of Windows 7 Activator
If you want to install this tool in your pc , your pc has minimum requirements :
– Microsoft .NET Framework 4.0 or higher .
Tag : microsoft toolkit download , activate windows by microsoft toolkit , activate MS Office by microsoft toolkit .
You can also use these tool to activate windows and Microsoft Office :
Download Microsoft Toolkit to activate Windows and MS Office- 4.01 / 5 5
Facebook Comments
Abstract
Impaired visceral white adipose tissue (WAT) metabolism has been implicated in the pathogenesis of several lifestyle-related disease states, with diminished expression of several WAT mitochondrial genes reported in both insulin-resistant humans and rodents. We have used rat models selectively bred for low- (LCR) or high-intrinsic running capacity (HCR) that present simultaneously with divergent metabolic phenotypes to test the hypothesis that oxidative enzyme expression is reduced in epididymal WAT from LCR animals. Based on this assumption, we further hypothesized that short-term exercise training (6 wk of treadmill running) would ameliorate this deficit. Approximately 22-wk-old rats (generation 22) were studied. In untrained rats, the abundance of mitochondrial respiratory complexes I–V, citrate synthase (CS), and PGC-1 was similar for both phenotypes, although CS activity was greater than 50% in HCR (P = 0.09). Exercise training increased CS activity in both phenotypes but did not alter mitochondrial protein content. Training increased the expression and phosphorylation of proteins with roles in β-adrenergic signaling, including β3-adrenergic receptor (16% increase in LCR; P < 0.05), NOR1 (24% decrease in LCR, 21% decrease in HCR; P < 0.05), phospho-ATGL (25% increase in HCR; P < 0.05), perilipin (25% increase in HCR; P < 0.05), CGI-58 (15% increase in LCR; P < 0.05), and GLUT4 (16% increase in HCR; P < 0.0001). A training effect was also observed for phospho-p38 MAPK (12% decrease in LCR, 20% decrease in HCR; P < 0.05) and phospho-JNK (29% increase in LCR, 20% increase in HCR; P < 0.05). We conclude that in the LCR-HCR model system, mitochondrial protein expression in WAT is not affected by intrinsic running capacity or exercise training. However, training does induce alterations in the activity and expression of several proteins that are essential to the intracellular regulation of WAT metabolism.
white adipose tissue (WAT) mass is linked to metabolic health and plays a critical role in the maintenance of whole body energy homeostasis (, ). Increased WAT mass, especially in visceral storage depots, is associated with a greater risk of metabolic disease and mortality (, ), whereas enlarged adipocyte cell size due to increased lipid content is linked to intrinsic cellular metabolic defects (, ). Conversely, small adipocytes may play a protective role against the increased risk of metabolic disease () because compared with larger adipocytes these cells have enhanced rates of glucose transport (, , , ). Although excessive lipid storage in visceral WAT depots is linked to metabolic abnormalities such as insulin resistance and impaired lipolysis (, ), the metabolic characteristics of WAT in differing metabolic phenotypes have not been well characterized.
Compared with other metabolically active tissues, the oxidative capacity of WAT is relatively low (), but essential cellular activities such as adipogenesis, lipogenesis, lipolysis, and fatty acid (FA) oxidation require large amounts of ATP (, , ). Given that WAT metabolism is altered in obesity and insulin resistance (, ) and the metabolic activity of most cells is highly dependent on mitochondrial content, impairments in the regulation of the adipocyte mitochondrial network may lead to dysfunctional WAT metabolism (). Indeed, diminished mitochondrial gene expression has been observed in WAT from insulin-resistant humans (, ) and rodents (, , ).
Through two-way artificial selection, we have generated animal models of high [high-intrinsic running capacity (HCR)] and low aerobic treadmill running capacity [low-intrinsic running capacity (LCR)] in the absence of exercise training (). Such selection has produced rats that simultaneously present with different metabolic and cardiovascular disease risk factors without the necessity for any environmental intervention (). Previously, we have shown that the metabolic characteristics of the skeletal muscle from these rats diverge substantially (, , ) and that exercise training ameliorates many of the adverse health features observed in the LCR rats (). Since exercise training is capable of reducing visceral WAT lipid content and adipocyte cell size (, , , ) while increasing the WAT expression of a number of mitochondrial proteins (, ), we hypothesized that the oxidative profile of visceral WAT would be lower in untrained LCR compared with HCR rats but that short-term exercise training would ameliorate this impairment. In line with this hypothesis, we aimed to determine whether differences in intrinsic running capacity or training state would affect the expression and activity of a number of proteins with important roles in WAT metabolism.
MATERIALS AND METHODS
Animal model.
This study was undertaken with the combined approval of the animal ethics committees from both the University of Michigan (Ann Arbor, MI) and California State University (Northridge, CA). Rat models for LCR and HCR were derived from genetically heterogeneous N:NIH stock (National Institutes of Health) rats by artificial selection for treadmill running capacity, as described previously (). Rats were housed in pairs in a temperature-controlled environment that provided a reverse 12:12-h light-dark cycle. Throughout the study, rats were given ad libitum access to standard rodent chow and water. Prior to the commencement of any experimental procedures, rats were allowed to acclimate to laboratory conditions for 1 wk.
Experimental design.
Age-matched pairs of ∼20-wk-old male LCR and HCR rats were randomly assigned to two groups: sedentary [LCR-SED (n = 10) and HCR-SED (n = 10)] or exercise trained [LCR-EX (n = 10) and HCR-EX (n = 10)]. Rats assigned to undergo exercise training completed a 6-wk (4 days/wk) incremental treadmill running protocol where all rats completed the same absolute cumulative running distance (∼10 km) (, ). Trained rats undertook their final exercise bout 48 h before the commencement of any experimental procedures.
Tissue collection and blood analyses.
Following a 5-h fast, blood samples were taken for the analysis of fasting blood glucose concentrations using a hand-held glucometer (Roche Diagnostics, Castle Hill, New South Wales, Australia). Serum was assessed for fasting insulin concentrations using a rat-specific ELISA (ALPCO diagnostics, Salem, NH) and for nonesterified FAs (NEFA) using a commercially available kit (WAKO Pure Diagnostics, Osaka, Japan). Rats were weighed and anesthetized using pentobarbital sodium (1 ml/kg body mass). Hindlimb skeletal muscles and epididymal fat pads were surgically excised, weighed, freeze-clamped in liquid nitrogen, and stored at −80°C for later analyses. Muscle data has been reported previously ().
Citrate synthase activity.
Approximately 100 mg of epididymal adipose tissue was visibly cleared of blood vessels and connective tissue and then mechanically homogenized in buffer [175 mM KCl and 2 mM EDTA (pH 7.4), 1:2 dilution] and centrifuged at 20,000 g for 15 min at 4°C. The infranatant was collected and assayed for citrate synthase activity, as described previously (). Protein concentration of the infranatant was determined using the bicinchoninic method (Pierce). Activity is expressed in nM·min−1·μg protein−1.
Immunoblotting.
Approximately 250–300 mg of epididymal adipose tissue was visibly cleared of blood vessels and connective tissue and then mechanically homogenized in buffer (50 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 50 mM NaF, 5 mM Na pyrophosphate, 10% glycerol, 1% Triton X-100, 10 μg/ml trypsin inhibitor, 2 μg/ml aprotinin, 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 1:8 dilution) and centrifuged at 20,000 g for 30 min at 4°C. Protein concentration of the infranatant was determined using the bicinchoninic method (Pierce). Adipose tissue lysates containing 10 μg of protein were prepared in 4× Laemmli buffer, subjected to SDS-PAGE, and then transferred to polyvinylidene difluoride membranes. A pooled lysate sample was prepared and included in each gel as an internal control for normalizing the data. Ponceau staining was used to confirm equal protein transfer. Membranes were then washed and blocked (5% nonfat dry milk or 5% BSA) for 1 h at room temperature prior to incubation with the appropriate antibodies. Membranes were incubated overnight at 4°C with primary antibodies specific for citrate synthase (CS; ∼52 kDa; Abcam, no. ab96600), mitochondrial respiratory complexes I, II, III, IV (subunit 4; COX-IV), and V of the electron transport chain (∼18, ∼25, ∼45, ∼15, and ∼52 kDa respectively; MitoSciences, nos. MA604 and MS407), uncoupling protein 1 (UCP1; Santa Cruz Biotechnology, no. sc6529), peroxisome proliferator-activated receptor-γ coactivator-1 (PGC-1; ∼100 kDa; Chemicon, no. ab3242), hormone-sensitive lipase (HSL; ∼88 kDa; Cell Signaling Technology, no. 4107), phospho-HSLS660 (), adipose triglyceride lipase (ATGL; ∼54 kDa; Cell Signaling Technology, no. 2138), phospho-ATGLS406 [∼54 kDa ()], β3-adrenergic receptor (β3-AR; ∼68 kDa; Santa Cruz Biotechnology, no. sc50436), perilipin 1 (PLIN1; ∼68 kDa; Sigma, P1873), comparative gene identification-58 (CGI-58; ∼42 kDa), FA-binding protein 4 (FABP4; ∼15 kDa; Abcam, no. ab37458), neuron-derived clone 77 (NUR77; ∼48 kDa; Santa Cruz Biotechnology, no. sc5569), neuron-derived orphan receptor 1 (NOR1; ∼68 kDa; Abcam, no .92777), GLUT4 (∼45 kDa; Abcam, no. ab654), AMP-activated protein kinase (AMPK)α (∼62 kDa; Cell Signaling Technology, no. 2532), AMPK phospho-Thr172 (∼62 kDa; Cell Signaling Technology, no. 2535), extracellular regulated kinase 1/2 (ERK1/2; ∼46 and ∼42 kDa; Cell Signaling Technology, no. 9102), phospho-ERK1/2T202/Y204 (∼46 and ∼42 kDa; Cell Signaling Technology, no. 9101), p38 mitotgen-activated protein kinase (p38 MAPK; ∼44 kDa; Cell Signaling Technology, no. 9212), phospho-p38 MAPKT180/Y182 (∼44 kDa; Cell Signaling Technology, no. 9211), c-Jun NH2-terminal ninase 1/2 (JNK1/2; ∼50 and ∼46 kDa; Cell Signaling Technology, no. 9252), or phospho-JNK1/2T183/Y185 (∼50 and ∼46 kDa; Cell Signaling Technology, no. 9251). Membranes were also probed with anti-α-tubulin (∼50 kDa; Cell Signaling Technology, no. 2144) or β-actin (∼42 kDa; Sigma-Aldrich) to confirm equal protein loading. After 1-h room temperature incubation in the appropriate secondary antibody, protein expression was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) and quantified by densitometry.
Statistical analyses.
All data are expressed as group means ± SE, unless specified otherwise. All data were analyzed using a two-way ANOVA, with running capacity and training state as fixed factors. Where main effects were considered significant, a Tukey test for multiple comparisons was conducted. Significance is reported where P < 0.05. All statistical analyses were completed using GraphPad Prism software.
RESULTS
Physiological parameters.
Data for body mass (BM) and fat pad mass have been reported previously (), with new statistical analyses presented here. Adipose tissue protein content is reported as an indirect marker of changes in adipocyte cellularity. Intrinsic running capacity and training status were main effects for BM (P < 0.0001 and P < 0.0001, respectively; Fig. 1A), fat pad mass (P < 0.0001 and P = 0.0002, respectively; Fig. 1B), and total tissue protein (P = 0.026 and P = 0.024, respectively; Fig. 1C). In addition, an interaction between running capacity and training status was observed for BM (P < 0.0001) and fat pad mass (P = 0.003). LCR rats were heavier than HCR rats with (33.4%, P < 0.0001) or without (26.6%, P < 0.0001) exercise training. The LCR-SED group was heavier than the LCR-EX group (31.1%, P < 0.0001), whereas there was no difference between HCR-SED and HCR-EX. Epididymal fat pad mass was greater in LCR than HCR rats with (62.5%, P < 0.0001) or without exercise training (50%, P < 0.0001). LCR-SED rats had heavier fat pads than LCR-EX rats (34.4%, P < 0.0001), whereas there was no difference between HCR-SED and HCR-EX rats. In line with this, total protein of WAT was lower in LCR-SED compared with HCR-SED rats (22.4%, P = 0.052), whereas adipose protein was 33.4% greater in LCR-EX compared with LCR-SED rats (P = 0.057). There were no differences in adipose protein between HCR-SED and HCR-EX or LCR-EX and HCR-EX rats.
Body mass (A), epididymal fat pad mass (B), and white adipose tissue (WAT) total protein (C) for low- (LCR; open bars) and high-capacity running rats (HCR; filled bars) with or without exercise training. Values are means ± SE; n = 8–10/group. Significance is reported where P < 0.05. *Main effect for running capacity; †main effect for training; ‡interaction between running capacity and training; adifferent from LCR-sedentary (SED); bdifferent from HCR-SED; cdifferent from LCR-exercise trained (EX); ddifferent from HCR-EX.
Intrinsic running capacity improved fasting blood glucose (main effect, P = 0.0002) and serum NEFA concentrations (main effect, P = 0.02; Table 1), but not fasting serum insulin levels. Compared with HCR-SED, LCR-SED rats had 8% higher blood glucose (P = 0.0008) and 37% higher NEFA concentrations (P = 0.003). Exercise training was a main effect for fasting serum NEFAs (P < 0.0001) and insulin concentrations (P < 0.0001), whereas there was a tendency for LCR-EX rats to have lower blood glucose concentrations than LCR-SED rats (8%; P = 0.11). Exercise training had little effect on blood glucose concentrations in HCR animals. Both LCR-SED and HCR-SED rats had higher serum insulin concentrations compared with LCR-EX (37%, P = 0.0004) and HCR-EX rats (38%, P = 0.0002), respectively. LCR-SED rats had 57% higher serum NEFA concentrations compared with LCR-EX rats (P < 0.0001), whereas training had little effect on NEFA concentrations in HCR animals.
Table 1.
| LCR-SED | HCR-SED | LCR-EX | HCR-EX | |
|---|---|---|---|---|
| Glucose, mM* | 6.2 ± 0.1b | 5.3 ± 0.1a | 5.7 ± 0.2 | 5.3 ± 0.2 |
| Insulin, ng/ml† | 0.5 ± 0.004c,d | 0.5 ± 0.02c,d | 0.3 ± 0.06a,b | 0.3 ± 0.07a,b |
| NEFA, mM*† | 0.38 ± 0.03b,c | 0.24 ± 0.02a | 0.16 ± 0.03a | 0.18 ± 0.02 |
Values are means ± SE; n = 7–10 rats/group. LCR, low-capacity running rats; HCR, high-capacity running rats; SED, sedentary; EX, exercise trained; NEFA, nonesterefied fatty acids.
CS activity.
CS activity was increased with exercise training (main effect, P = 0.01; Fig. 2A) and tended to be increased in rats with HCR (main effect, P = 0.09). CS activity was 58% higher in epididymal fat pads from LCR-EX compared with LCR-SED rats (P = 0.08).
A: citrate synthase activity of epididymal WAT from LCR (open bars) and HCR rats (filled bars) with or without exercise training. Values are means ± SE; n = 8–10/group. Significance is reported where P < 0.05. †Main effect for training; adifferent from LCR-SED; ddifferent from HCR-EX. B: representative immunoblots of mitochondrial proteins. CI, respiratory complex I; CII, respiratory complex II; CIII, respiratory complex III; CIVIV, respiratory complex IV, subunit IV; PGC-1, peroxisome proliferator-activated receptor-γ coactivator-1.
Mitochondrial protein content.
Under equal protein loading conditions (10 μg of total protein), the contents of tricarboxylic acid (TCA) cycle (CS), oxidative phosphorylation (electron transfer complexes I–V), and mitochondrial biogenesis (PGC-1) proteins were similar between LCR and HCR rats independent of exercise training status (Fig. 2B). UCP1 was not detected in any sample (data not shown).
Intracellular regulators of lipolysis.
β3-AR expression was increased with exercise training (main effect, P = 0.03; Fig. 3A), whereas running capacity showed a tendency to increase this parameter (P = 0.09). There was a significant interaction between running capacity and exercise training (P = 0.006). The expression of the β3-AR was 17% greater in HCR-SED compared with LCR-SED rats (P = 0.006) and 18% greater in LCR-EX compared with LCR-SED rats (P = 0.004).The phosphorylation of ATGL at Ser406 and HSL at Ser660 was assessed as surrogate markers of their activity (, ). ATGL Ser406 phosphorylatation and total ATGL protein content were increased by both running capacity (P = 0.02 and P = 0.003, respectively) and exercise training status (P = 0.0005 and P = 0.03, respectively; Fig. 3, B and C). Post hoc analyses revealed a 25% increase in ATGL Ser406 phosphorylation in HCR-EX compared with HCR-SED rats (P = 0.01; Fig. 3B). Total ATGL expression was 17% greater in HCR-EX compared with LCR-EX rats (P = 0.04; Fig. 3C) and the ratio of ATGL Ser406 to total ATGL was not different. Total HSL protein remained similar for all groups, although there was a tendency for HSL Ser660 phosphorylation to be decreased in both LCR-EX and HCR-EX rats with training (main effect, P = 0.09). There was no difference in the ratio of HSL Ser660 phosphorylation to total HSL protein (data not shown). The content of PLIN1 (which controls lipolysis by regulating protein-protein interactions at the surface of lipid droplets, thereby facilitating access of lipases to their substrates) was increased by training (P = 0.02; Fig. 3D), and an interaction was observed between training and running capacity (P = 0.003). Post hoc analyses revealed that PLIN1 expression was 19% greater in LCR-SED compared with HCR-SED rats (P = 0.03) and 25% greater in HCR-EX compared with HCR-SED rats (P = 0.002). The protein content of CGI-58 (which binds to and activates ATGL triglyceride lipase activity) was increased by 15% in HCR-SED compared with LCR-SED rats and 15% greater in LCR-EX compared with LCR-SED rats, although neither of these values attained statistical significance (P = 0.08 and P = 0.1, respectively; Fig. 3E). There were main effects for both running capacity and exercise training for CGI-58 protein content (P = 0.04 and P = 0.03, respectively; Fig. 3E). No difference in FABP4 expression was observed between groups (data not shown).
Representative immunoblots and relative protein expression of β3-adrenergic receptor (β3-AR; A), phospho- total adipose triglyceride lipase (ATGL)S406 (B), ATGL (C), perilipin 1 (PLIN1; D), and comparative gene identification-58 (CGI-58; E) in epididymal WAT from LCR (open bars) and HCR rats (filled bars) with or without exercise training. Values are means ± SE; n = 8–10/group. Significance is reported where P < 0.05. *Main effect for running capacity; †main effect for training; ‡interaction between running capacity and training; adifferent from LCR-SED; bdifferent from HCR-SED; cdifferent from LCR-EX; ddifferent from HCR-EX. AU, arbitrary units.
NOR1, NUR77, and GLUT4 expression.
A main effect of exercise training was observed for NOR1 expression (P < 0.0001; Fig. 4A). Post hoc analyses revealed that NOR1 expression was increased by 24% in LCR-SED compared with LCR-EX rats (P = 0.008) and 21% in HCR-SED compared with HCR-EX rats (P = 0.01). Intrinsic running capacity did not influence the expression pattern of NOR1, whereas the expression of NUR77 was similar in LCR and HCR rats with or without exercise training (results not shown). The expression of GLUT4 was elevated with high intrinsic running capacity (P < 0.0001; Fig. 4B) and with exercise training (P = 0.007), with post hoc analyses revealing that GLUT4 protein content was increased by 16% n HCR-EX compared with HCR-SED rats (P = 0.01). Compared with LCR-EX rats, GLUT4 protein content was 22% greater in HCR-EX rats (P = 0.0007).
Representative immunoblots and relative protein expression of neuron-derived orphan receptor 1 (NOR1; A) and glucose transporter 4 (GLUT4; B) in epididymal WAT from LCR (open bars) and HCR rats (filled bars) with or without exercise training. Values are means ± SE; n = 8–10/group. Significance is reported where P < 0.05. *Main effect for running capacity; †main effect for training; adifferent from LCR-SED; bdifferent from HCR-SED; cdifferent from LCR-EX; ddifferent from HCR-EX.
Office 2010 Toolkit And Ez Activator 2 2 3 Rat Rod
Stress kinase activation.
We investigated several stress-activated kinases to determine their involvement in the adaptive response of WAT metabolism to exercise training. Total p38 MAPK expression was reduced with training (P = 0.03; Fig. 5A), whereas there was a tendency for phosporylation of p38 MAPK on the Thr180 and Tyr182 residues to be reduced by training (P = 0.07). There was a main effect of exercise training on the ratio of phospho-p38 MAPKT180/Y182 to total p38 MAPK (P = 0.03). No differences were observed in total JNK1/2 expression; however, training increased phospho-JNK1/2T183/Y185 (P = 0.02) and the ratio of phospho-JNK1/2T183/Y185 to total JNK1/2 (P = 0.002; Fig. 5B). No differences were observed in total ERK1/2 expression (Fig. 5C), although a significant main effect of running capacity was observed for phospho-ERK1/2T202/Y204 (P = 0.03). There was also a tendency for the ratio of phospho-ERK1/2T202/Y204 to total ERK1/2 to be affected by running capacity (LCR > HCR, P = 0.06). No differences were observed in total or phospho-AMPKT172 expression, or the ratio of phospho-AMPKT172 to total AMPK expression (data not shown).
Representative immunoblots and relative protein expression of phospho-p38 MAPKT180/Y182 and total p38 MAPK (A), phospho-JNK1/2T183/Y185 and total JNK1/2 (B), and phospho-ERK1/2T202/Y204 and total ERK1/2 in epididymal WAT (C) from LCR (open bars) and HCR rats (filled bars) with or without exercise training. Values are means ± SE; n = 8–10/group. Significance is reported where P < 0.05. †Main effect for training; adifferent from LCR-SED; cdifferent from LCR-EX; ddifferent from HCR-EX.
DISCUSSION
Using a rat model of divergent running ability, we present novel data demonstrating that 1) the mitochondrial protein content of visceral WAT is not related to intrinsic exercise capacity, 2) a short-term (6 wk) program of endurance exercise training does not modulate visceral WAT mitochondrial protein expression (despite training-induced increases in citrate synthase activity), 3), intrinsic running capacity and training status are associated with the differential WAT expression of several key lipolytic proteins, and 4) irrespective of intrinsic running capacity, exercise training induces alterations in the activity and expression of a number of proteins essential to the intracellular regulation of WAT lipid metabolism.
In humans, low aerobic capacity is a strong predictor of early mortality (, ). This important clinical association suggests that the capacity for oxygen metabolism is the underlying determinant of the divide between complex disease and health (aerobic hypothesis; see Ref. ). We used artificial selection for aerobic capacity as an unbiased test of this theory. Along with cardiovascular disease risk () and reduced lifespan (), LCR rats express a number of characteristics common to metabolic disease phenotypes, such as increased body mass and adiposity (, ), hyperinsulinemia (, , ), and impaired glucose tolerance (, , ). In contrast, HCR rats live 6–8 mo longer () and present with superior metabolic health characterized by resistance to weight gain in the face of a high-fat diet (, ) and an increased capacity for the uptake and oxidation of glucose (, , ) and FAs (, , , ). Although the differential expression of these key characteristics appears to be linked to the oxidative capacity of the skeletal muscle (), less is known about the metabolic characteristics of the adipose tissue from these divergent aerobic phenotypes.
We have shown previously that the skeletal muscle of LCR rats contains fewer mitochondria than HCR rats (, ). Furthermore, we have also observed impaired β-adrenergic signaling and lipolysis in the muscle of LCR rats (, ). Given the divergent metabolic characteristics observed in LCR and HCR rats and the importance of WAT in regulating circulating concentrations of FAs and glucose (, , ), we sought to determine whether differences similar to those seen in muscle could also be identified in the WAT. Unlike previous investigations that reported reduced expression of a selection of mitochondrial genes and proteins in the visceral WAT of insulin-resistant rodents (, ) and humans with type 2 diabetes (), we did not detect differences in the expression of the key proteins involved in oxidative phosphorylation and the tricarboxylic acid (TCA) cycle in visceral WAT from LCR and HCR phenotypes (Fig. 2B). Although we cannot rule out the possibility that differences in mitochondrial protein expression were masked due to equal amounts of protein being analyzed in each experiment (particularly since LCR-SED rats have less protein per gram of adipose tissue compared with the other 3 groups; Fig. 1C), the level of expression of all mitochondrial proteins measured was consistent for both sedentary and exercise-trained cohorts, suggesting that the abundance of oxidative enzymes in visceral WAT may not be an important factor for determining running capacity and the associated phenotypes of the LCR-HCR rat model system.
Chronic β-adrenergic stimulation as a result of increased physical activity (, ) or pharmacological activation () has been shown to enhance the oxidative capacity of WAT by upregulating the expression of genes involved in oxidative phosphorylation and fat oxidation. Although the training program employed in this study was successful in ameliorating many of the metabolic differences observed in the skeletal muscle of LCR compared with HCR rats (), including a reduction in body mass and adiposity (Fig. 1), it had little effect on oxidative enzyme expression in the WAT of either phenotype, an observation that is consistent with previous findings from our laboratory in human subcutaneous WAT (). Although mitochondrial protein content was not different, we observed a marked training-induced increase in citrate synthase activity in both LCR and HCR phenotypes (Fig. 2A). Although this might appear to be in contrast to our earlier findings (especially given that citrate synthase protein expression was unchanged), it is important to note that citrate is essential for replenishing the extramitochondrial pool of acetyl-CoA, a substrate essential to de novo lipogenesis (). Furthermore, lipogenic pathways are energetically costly processes. Therefore, although training may not have altered the amount of mitochondrial protein, changes in cellular energy needs in response to physiological demand are likely to have induced tighter allosteric control and a number of posttranslational changes that increase the maximal capacity of mitochondrial enzymes involved in the different energy-producing pathways (). A limitation of the current study is the absence of additional measures representing the coordinated activities of β-oxidation, the TCA cycle, and oxidative phosphorylation.
Glucose incorporation into triglycerides (TG) is an essential component of lipid synthesis, and intracellular glucose availability in adipocytes is dependent on plasma membrane glucose transport efficiency [a process that is associated directly with the intracellular pool of GLUT4 (, , )]; training-induced increases in WAT GLUT4 expression may be associated with an increased capacity for WAT TG synthesis. Indeed, adipose-specific GLUT4 overexpression induces an increase in the capacity for TG synthesis via both reesterification and de novo lipogenic pathways, leading to an increase in total adipose mass in sedentary animals (, ). Although we were unable to measure lipogenic activity in the present study, we demonstrate that both running capacity and training status affect GLUT4 content in visceral WAT (Fig. 4B), with LCR rats having reduced WAT GLUT4 content compared with HCR rats. Whether or not other adipose depots show similar changes remains to be determined. Either way, differences in adipose GLUT4 content in both sedentary and trained animals could have important implications on whole body adiposity in these divergent rat phenotypes (). It is also worth noting that exercise training has been reported to increase subcutaneous WAT GLUT4 expression in type 2 diabetic humans (). Whether the same changes are observed in human visceral WAT is unclear, since subcutaneous and visceral adipose depots may have distinct responses to exercise (). Similarly, it is possible that the different visceral adipose depots may respond disparately to training. Thus, another limitation of the current study is limiting our analyses to the epididymal WAT depot.
TG synthesis is tightly coupled with TG catabolism; therefore, it is not surprising that exercise training induces an increase in the capacity of both basal and catecholamine-stimulated WAT lipolysis (). This is attributed predominantly to repeated transient elevations in circulating catecholamines acting via β-adrenergic signaling pathways (). The β3-AR plays an important role in regulating energy balance, particularly in WAT (). The expression of the β3-AR was reduced in LCR-SED compared with HCR-SED rats (Fig. 3A), which agrees with studies in both obese rodents (, ) and humans () that report impaired β-adrenergic signaling as an important factor in obesity development. Indeed, functional β-adrenergic signaling is essential for obesity resistance (). In line with this, exercise training “rescued” the reduction in β3-AR in LCR-EX rats (Fig. 3A) while concomitantly reducing fat pad and total body mass (Fig. 1). Conversely, training did not effect β3-AR levels in HCR-EX rats (Fig. 3A), nor did it affect fat pad or total body mass (Fig. 1). These findings are similar to our previous observation of impaired β-adrenergic signaling in the skeletal muscle of LCR compared with HCR rats (), an impairment that is also ameliorated with exercise training ().
β3-AR stimulation by catecholamines increases lipolysis by activating protein kinase A (PKA), which phosphorylates both ATGL () and HSL () to increase lipase activity. Furthermore, PKA phosphorylation of PLIN1 facilitates the dissociation of CGI-58 from PLIN1, thereby allowing CGI-58 to interact with ATGL to maximally activate lipolysis (). Given that the primary hypotheses of the current study were mitochondrially focused, we did not measure lipolysis directly. Instead, we determined the phosphorylation state of ATGL and HSL at key activating serine residues as well as the protein abundance of other key lipolytic proteins. ATGL Ser406 phosphorylation and total ATGL content were increased in HCR vs. LCR rats and by exercise training in both groups (Fig. 3, B and C). Similarly, CGI-58 was increased in HCR vs. LCR rats and by exercise training in both groups (Fig. 3E). Based on our knowledge of protein function and results from knockout mice studies (), the changes reported herein would predict increased lipolysis in high-capacity runners and following exercise training. Such a response would match fatty acid availability with the increased fatty acid oxidation capacity/rates observed in HCR compared with LCR rats (, , ) and is consistent with an endurance-trained individual's reliance on FA as an energy source ().
Previous investigations have reported links between β-adrenergic signaling, the expression of the orphan nuclear receptor NOR1, and whole body lipid and carbohydrate metabolism (). Although its targets are largely unknown, NOR1 is purported to play an important role in regulating oxidative metabolism and glucose transport in a number of tissues (). Notably, NOR1 is a cAMP-dependent target of PKA that is upregulated upon HSL and ATGL inhibition (, ). We have shown recently that NOR1 expression is reduced in the skeletal muscle of LCR compared with HCR rats and have suggested that this may be linked to the oxidative capacity of the tissue (). Here, we demonstrate that the difference in NOR1 expression is tissue specific, as LCR-SED and HCR-SED rats displayed a similar abundance of NOR1 in WAT. This is incongruous with human studies that demonstrate that NOR1 is more highly expressed in the WAT of obese compared with healthy humans (). However, following weight loss the expression of NOR1 in human WAT is “normalized” to levels similar to healthy control subjects (). Similarly, we have observed a reduction in NOR1 expression of a similar magnitude for both LCR and HCR rats following exercise training (24 and 21% respectively; Fig. 4A). Taken collectively, our observation of reduced NOR1 expression following exercise training in both LCR and HCR rats suggests a principal role for NOR1 in the metabolic regulation of WAT. Ftir software download.
Given the role of β-adrenergic signaling in WAT metabolism and our finding that the expression of several proteins downstream of β-adrenergic stimulation is dependent on running capacity and/or training status (Fig. 5), we sought to characterize the phosphorylation of several stress kinases that are purported to interact with β-adrenergic signaling pathways to influence intracellular metabolism (, , ). To our knowledge, we are the first to report that a short-term exercise training program induces distinct changes in the activity of the stress kinases p38 MAPK and JNK in visceral WAT. Both p38 MAPK and JNK are activated by FAs (, ), and both are increased in response to elevated lipolytic activity (). Importantly, the β-adrenergic activation of p38 MAPK has been shown to occur through cAMP-dependent mechanisms involving PKA (), and both p38 MAPK and JNK phosphorylation are known to be elevated in visceral WAT during human obesity (). Here, we have observed a modest reduction in the phosphorylation of p38 MAPK in visceral WAT following exercise training (Fig. 5A), a finding that is consistent with observations in human and rodent skeletal muscle, where training-induced attenuation of resting p38 MAPK activity is associated with the adaptive response to exercise training (, ). It is also worth noting that p38 MAPK is a known activator of PGC-1α (and therefore mitochondrial biogenesis) (, ), and as such, training-induced reductions in p38 MAPK activity may partially explain why we did not see changes in mitochondrial protein expression in the current study. In contrast to the findings for p38 MAPK, phosphorylation of JNK was elevated as a result of exercise training (Fig. 5B). This is also consistent with findings in skeletal muscle () but inconsistent with reports that diet-induced elevations in JNK phosphorylation are attenuated by swim training in obese rats (, ). Although the individual roles of JNK and p38 MAPK in adipocyte metabolism are no doubt complex, our findings indicate that in WAT both kinases are exercise responsive (in a manner similar to findings in skeletal muscle), thus implicating them in the array of signaling responses associated with changes in energy demand.

In summary, we have investigated the relationship between exercise capacity and WAT metabolism in relation to whole body metabolic health. We have presented novel data showing that the content of selected mitochondrial proteins in visceral WAT does not differ in relation to intrinsic exercise capacity or exercise training status, and as such, we propose that tighter allosteric control and/or posttranslational regulation of oxidative enzymes may be important for coordinating aerobic energy metabolism in WAT. We also show that running capacity and training state regulate the expression and activity of a number of proteins downstream of β-adrenergic signaling pathways, particularly those that are essential to WAT lipolysis. We suggest that training-induced changes in the expression of the orphan nuclear receptor NOR1 and the activities of the stress kinases p38 MAPK and JNK may be important factors for determining the expression and activity of metabolic proteins following exercise training. Further studies are necessary to provide mechanistic support for these purported relationships.
GRANTS
This study was funded in part by a National Heart Foundation Grant-in-Aid (G 09M 4348) to J. A. Hawley. The LCR-HCR rat model system was funded specifically by National Center for Research Resources Grant R24 RR017718; and is currently supported by the Office of Research Infrastructure Programs/OD Grant ROD012098A (to L. G. Koch and S. L. Britton) from the National Institutes of Health.
DISCLOSURES
The authors declare no conflict of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
E.J.S., S.J.L., D.A.R., M.J.W., B.B.Y.I., L.G.K., S.L.B., and J.A.H. contributed to the conception and design of the research; E.J.S., S.J.L., D.A.R., and B.B.Y.I. performed the experiments; E.J.S. and S.J.L. analyzed the data; E.J.S. interpreted the results of the experiments; E.J.S. prepared the figures; E.J.S. drafted the manuscript; E.J.S., M.J.W., L.G.K., S.L.B., and J.A.H. edited and revised the manuscript; E.J.S., S.J.L., D.A.R., M.J.W., L.G.K., S.L.B., and J.A.H. approved the final version of the manuscript.
REFERENCES